Now that hundreds of millions of doses of COVID-19 vaccines have been administered around the world, an abundance of safety data has been collected. While this data shows that COVID-19 vaccines are safe and effective at helping prevent infection, extremely rare adverse reactions can occur.
Myocarditis and pericarditis are conditions that have occurred following COVID-19 vaccination in a very small number of people. Most cases are mild, and individuals often recover on their own or with minimal treatment. Myocarditis and pericarditis are much more common if you get COVID-19. The risks to the heart from COVID-19 infection can be more severe.
What is myocarditis and pericarditis?
Myocarditis is a rare condition that causes inflammation of the heart muscle. Pericarditis is inflammation of the outer lining of the heart. These responses have been reported as very rare adverse reactions to coronavirus mRNA vaccines. They are not unique to COVID-19 vaccination
Myocarditis and pericarditis are typically caused by infection with a virus, including viruses that cause the common cold. The cause of these conditions is not always known.
What are the symptoms?
- Chest pain
- Shortness of breath
- Fever
What is the association with COVID-19 vaccines?
Rare cases of inflammation of the heart have occurred after the second dose of mRNA vaccines (Moderna and Pfizer-BioNTech). Symptoms typically occur within a week after the second dose of the vaccines. The association is so rare that, according to a study in Israel, there were only 54 reported cases of myocarditis among more than 2.5 million people vaccinated—or 0.002%. In July 2021, there were 1,000 reported and 300 confirmed cases of myocarditis or pericarditis in the U.S.
The following guidance has been developed jointly by the Australian Technical Advisory Group on Immunisation (ATAGI), the Cardiac Society of Australia and New Zealand (CSANZ), the Royal Australian College of General Practitioners (RACGP), the Australian College of Rural and Remote Medicine (ACRRM), the Australasian College for Emergency Medicine (ACEM) and the Paediatric Research in Emergency Departments International Collaborative (PREDICT).
Key Points
- A small increased risk of pericarditis and/or myocarditis has been observed in people who have received an mRNA COVID-19 vaccine (including Comirnaty (Pfizer) and Spikevax (Moderna), compared to unvaccinated people.
- COVID-19 itself is associated with a substantially higher risk of myocarditis and other cardiac complications compared to vaccination.
- ATAGI, CSANZ, RACGP, ACCRM, ACEM and PREDICT therefore emphasise that the overwhelming benefits of vaccination in protecting against COVID-19 greatly outweigh the rare risk of myocarditis and/or pericarditis. Comirnaty and Spikevax continue to be recommended for all people aged 12 years and above.
- Pericarditis and myocarditis after mRNA COVID-19 vaccines have been reported most commonly in males under 30 years of age, and most commonly after the second vaccine dose. Most myocarditis and pericarditis linked to mRNA vaccination has been mild and patients have recovered quickly. Longer-term follow-up is ongoing.
- Vaxzevria (AstraZeneca) is not associated with an increased risk of myocarditis and/or pericarditis. While cases have been reported after this vaccine, they have not been reported more frequently than what is expected in the absence of vaccination (the ‘background rate’).
- Pre-existing cardiac conditions are not regarded as a contraindication to vaccination.
- People with a history of any of the following conditions can receive an mRNA vaccine (e.g. Comirnaty or Spikevax) but should consult a GP, immunisation specialist service or cardiologist about the best timing of vaccination and whether any additional precautions are recommended:
- Recent (i.e., within the last 3 months) myocarditis or pericarditis
- Acute rheumatic fever or acute rheumatic heart disease (i.e., with evidence of active inflammation)
- Acute decompensated heart failure
- Symptoms of myocarditis or pericarditis typically appear within 1-5 days of an mRNA vaccine dose and may include chest pain, palpitations (irregular heartbeat), syncope (fainting) or shortness of breath. People who experience any of these symptoms after having an mRNA COVID-19 vaccine should seek prompt medical attention.
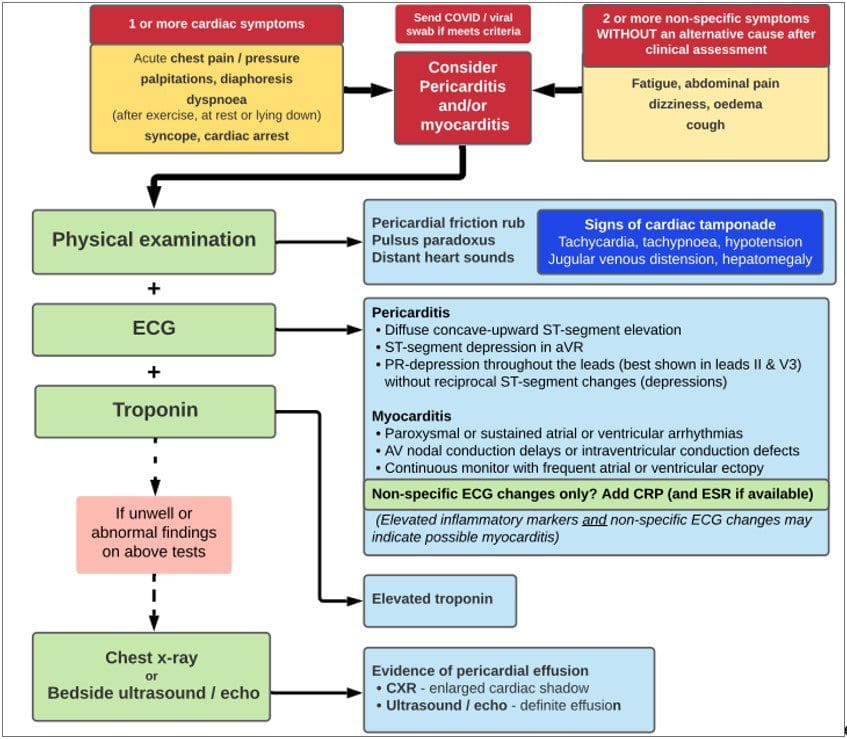
- Initial assessment and investigation can be done in a general practice or an ambulatory outpatient cardiology setting for patients who are not acutely unwell and when initial investigation results can be obtained and reviewed within 12 hours. Based on a clinical severity and risk assessment, some patients may require review in an emergency department.
- Initial investigations should include ECG and blood troponin levels. A chest X-ray, and other investigations for other differential diagnoses should be undertaken as clinically indicated.
- Emergency department guidance on the assessment of children or adolescents presenting with chest pain following an mRNA COVID-19 vaccine is available at https://www.predict.org.au/mrna-chest-pain-guideline/.
- Future vaccine dose recommendations vary depending on investigation results.
- Further doses of an mRNA COVID-9 vaccine can be given to people who have been investigated for pericarditis but who had normal ECG, troponin and inflammatory markers, and who have been symptom-free for at least 6 weeks. This includes people with a clinical diagnosis of pericarditis despite normal investigations.
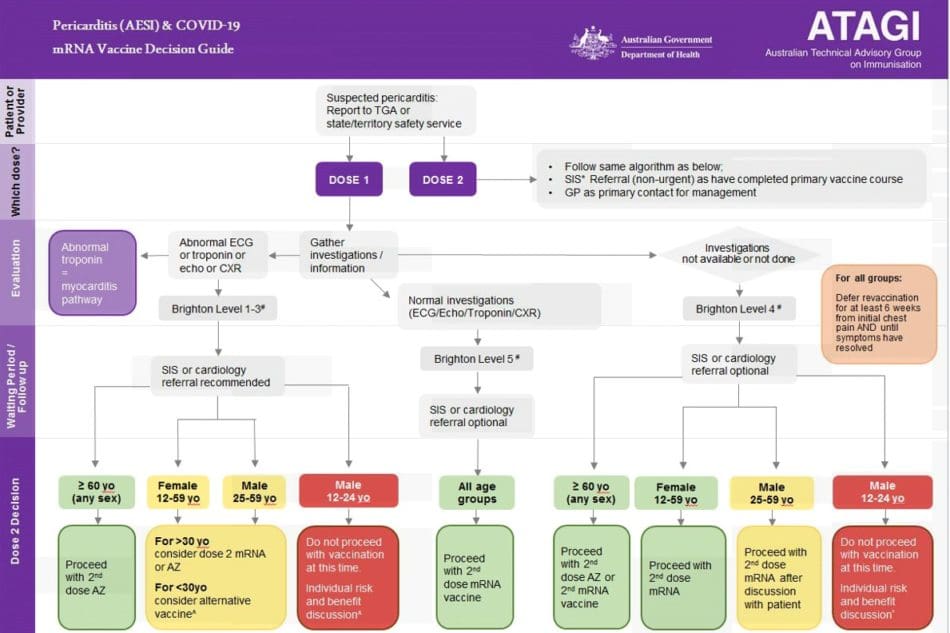
- For people with suspected or proven pericarditis and abnormal investigation results, the need and choice of further doses is informed by age and sex (refer to Figure 2 for future dose recommendations).
- People who have had confirmed myocarditis attributed to a dose of Comirnaty or Spikevax should defer further doses of an mRNA COVID-19 vaccine and if they are > 18 years can consider Vaxzevria on a case-by-case basis, after they have recovered from their symptoms.
Table 1: Reporting rates of myocarditis (per 1 million doses administered) to the United States VAERS after mRNA COVID-19 vaccines, 7-day risk period (N = 935).16
| Pfizer | Moderna | ||
| (All) | (All) | ||
Ages | Dose 1 | Dose 2 | Dose 1 | Dose 2 |
12-15 | 2.3 | 21.5 | 0.0 | not calculated |
16-17 | 2.8 | 37.4 | 0.0 | not calculated |
18-24 | 1.2 | 18.1 | 3.1 | 20.7 |
25-29 | 0.7 | 5.7 | 1.8 | 11.2 |
30-39 | 0.6 | 2.8 | 1.4 | 3.6 |
40-49 | 0.2 | 1.5 | 0.2 | 2.1 |
50-64 | 0.3 | 0.4 | 0.5 | 0.5 |
65+ | 0.1 | 0.2 | 0.0 | 0.3 |
Updated data from the MHRA is available at https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting.
Up-to-date data on cases and rates of myocarditis and pericarditis reported to the Australian Therapeutic Goods Administration is available at https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report.
Most myocarditis cases linked to mRNA vaccination have required hospitalization, with the majority responding well to standard treatment and having a mild and self-limiting course.3,8,13. In a case series of 25 children aged 12-18 with probable myopericarditis after mRNA COVID-19 vaccination, 92% had a normal cardiac function on echocardiogram.21 Pericarditis cases are often managed in primary and/or ambulatory care and also have a short, self-limiting course
Recommendations
ATAGI, CSANZ, RACGP, ACRRM, ACEM and PREDICT emphasize the overwhelming benefits of vaccination using an mRNA vaccine to protect individuals from COVID-19 and its serious outcomes such as hospitalization and death, as well as the wider benefits of reducing the spread of the disease in the community, greatly outweigh the rare risk of myocarditis or pericarditis after vaccination.
Comirnaty and Spikevax continue to be recommended for all people aged ≥ 12 years.
Advice for people with a history of cardiac conditions
Comirnaty and Spikevax continue to be recommended to prevent COVID-19 in people with a history of chronic cardiovascular conditions, including coronary artery disease, myocardial infarction, stable heart failure, arrhythmias, rheumatic heart disease (RHD), Kawasaki Disease, congenital heart disease, cardiomyopathy, or a cardiac transplant, and in people with implantable cardiac devices. No specific precautions are recommended for people in these groups. There is no current data suggesting that their risk of developing myocarditis or pericarditis after vaccination is any higher than for the general population.
People with a history of any of the following conditions can receive Comirnaty or Spikevax but should consult a GP, immunization specialist service, or cardiologist about the best timing of vaccination and whether any additional precautions are recommended:
- Current or recent (i.e., within past 3 months) myocarditis or pericarditis due to causes other than vaccination (see Future Does Recommendations)
- Acute rheumatic fever or acute rheumatic heart disease (i.e., with evidence of active inflammation)
- Acute decompensated heart failure
These patients should be counseled about the symptoms to look out for after vaccination, and some may be advised by their cardiologist to schedule a routine visit with their general practitioner a few days after vaccination to screen for any concerning symptoms or signs.
Vaxzevria (AstraZeneca) can be considered as an alternative vaccine in individuals > 18 years, particularly in people aged ≥ 60 years.
What to look out for after vaccination
During the consent process, all people who receive Comirnaty or Spikevax should be advised of the very rare risk of myocarditis and/or pericarditis after vaccination and be advised of the symptoms in Table 2, and what to do if they develop.
Symptoms typically start within a few days after vaccination (median 2 days).15 People who experience any of these symptoms after receiving Comirnaty or Spikevax should seek prompt medical attention. People who feel well and do not have any of these symptoms after vaccination can continue with their usual physical activity and do not routinely need to avoid physical exertion.
People who have underlying heart dysfunction should seek medical attention for new-onset or worsening of pre-existing symptoms following vaccination.
Table 2: Symptoms and signs of myocarditis or pericarditis
| Myocarditis | Pericarditis |
Symptoms | Chest pain, pressure or discomfort Palpitations Shortness of breath Non-specific symptoms e.g. fatigue
| Chest pain which may be sharp, worse when lying down, and alleviated when sitting up and leaning forward Pain on deep inspiration
|
Signs | May have normal examination Tachycardia Severe myocarditis: signs of cardiac dysfunction e.g. third heart sound, oedema | Pericardial rub on auscultation |
Assessment of possible myocarditis or pericarditis in a primary care setting
Initial investigations can be performed in the primary care setting, based on clinical judgment, if:
- the patient is not acutely unwell and has mild symptoms
- the referring practice can obtain and review all the results of initial investigations within 12 hours. If required, contact your local pathology service before sending the patient for blood tests to ensure this.
Patients with possible myocarditis and/or pericarditis should immediately be referred to ED if any of the following apply:
- they are acutely unwell as assessed by the clinician (any age)
- they have any chest pain and are aged ≥ 30 years
- they have abnormal ECG findings (refer to Table 2 below)
- initial investigations cannot be performed and reviewed within 12 hours
Refer to Table 3 below for the initial investigations recommended for the evaluation of myocarditis or pericarditis.
Table 3: Initial diagnostic evaluation of myocarditis and pericarditis
Investigation | Findings | |
| Myocarditis | Pericarditis |
12- lead ECG | · ST or T-wave abnormalities#, Q waves · Premature atrial complexes · Premature ventricular complexes · Can be normal | · Widespread ST elevation (typically concave up) · PR depression · Small QRS (reflecting pericardial effusion) · Can be normal or atypical |
Troponin | · Commonly raised, however absence of elevation* does not exclude myocarditis | · May be increased (suggestive of myopericarditis) |
Inflammatory markers: CRP, ESR | · Commonly raised (although nonspecific) | · Commonly raised (although nonspecific) |
Chest X-ray (PA) | · Heart size can be normal or enlarged (in children this is defined as cardiothoracic ratio >0.5) | · Typically normal · Rarely, large pericardial effusion can lead to cardiomegaly |
#N.B. T wave inversion in anterior leads can be normal in people aged ≤ 16 years
* If ongoing clinical concern, could consider repeating troponin in 12 hours
Referral & Management
Patients with confirmed myocarditis or pericarditis may require referral to a cardiologist for advice regarding management (depending on the patient’s location a telehealth consult may be appropriate with a cardiologist and/or medical retrieval team).
Further investigations may be required, including:
- Investigations to exclude other causes e.g., viral illness
- Echocardiogram
- Coronary angiography or CT coronary angiogram for selected patients who may present with features indistinguishable from acute coronary syndrome
- Cardiac MRI
- Endomyocardial biopsy is rarely indicated (as determined by cardiologist)
Treatment of myocarditis and pericarditis is determined on a case-by-case basis and often supportive treatment is all that is required.22
Patients with confirmed myocarditis should be admitted to the hospital for cardiac monitoring (ideally continuous ECG monitoring), until the cardiac biomarker levels have peaked and symptoms have resolved.
Follow up in the community
- People for whom management in the community is advised should be reviewed by their general practitioner every 1-2 days.
- Advise patients to avoid high-intensity exercise or competitive sports until resolution of symptoms and ECG changes, and normalization of cardiac function.
- After a diagnosis of myocarditis and/or pericarditis, cardiology follow-up will be required for at least 12 months. A repeat ECG and echocardiogram are likely to be required.
Follow up of patients with normal investigations
- Patients with minor symptoms, normal ECG, and no elevation in troponin and/or inflammatory markers can be monitored in the community with a GP review every 1-2 days.
- Investigations should be repeated if symptoms are persistent.
- Advise patients to avoid high-intensity exercise or competitive sports until symptoms have resolved.
- Clinical judgment should be used as to the need for specialist consultation. Refer to ED or discuss with a cardiologist if there are any abnormalities on repeat investigations, or if any concerning symptoms (even if investigations are normal).
Assessment of possible myocarditis or pericarditis in an emergency department setting
Chest pain is a common emergency department presentation in adults and has a broad differential. Adults who present with chest pain following mRNA COVID-19 vaccine should be investigated for other causes of chest pain (such as acute coronary syndrome) as indicated, based on their history and examination findings.
Chest pain is less common in children and adolescents. Figure 1 outlines the recommended investigations in children and adolescents with possible vaccine-induced myocarditis/pericarditis, developed by the Paediatric Research in Emergency Departments International Collaborative, ACEM, ATAGI, New Zealand Immunisation Advisory Centre and Cardiac Society of Australia and New Zealand and is available at: https://www.predict.org.au/mrna-chest-pain-guideline
Future dose recommendations
Recommendations regarding future COVID-19 vaccine doses will depend on the specific diagnosis (i.e., myocarditis or pericarditis), level of certainty of the diagnosis, and the patient’s age. Options include deferring any further COVID-19 vaccine until further information is available, choosing an alternate vaccine formulation, or proceeding with further mRNA COVID-19 vaccine doses.
Myocarditis
As the more serious adverse event following immunization (AEFI), myocarditis should be discussed with a specialist immunization service (SIS) and/or cardiologist prior to administering a subsequent COVID-19 vaccine dose.
Pericarditis
Figure 2 outlines the approach to revaccination in people with pericarditis attributed to an mRNA COVID-19 vaccine. Referral to a specialist immunization service (SIS) or cardiologist is not always required.
People who have had a clinical diagnosis of pericarditis following an mRNA vaccine but who have normal investigations (i.e., ECG, echocardiogram, troponin, and chest X-ray) can receive further doses after full recovery. Patients should be symptom-free for at least 6 weeks. The need and choice of further doses are informed by investigation findings, age, and sex.
Prognosis and long term follow up
There are currently limited available data on the long-term outcomes of people who have had myocarditis and/or pericarditis after an mRNA COVID-19 vaccine. Studies monitoring outcomes are ongoing in the United States and Canada. Short to medium-term follow-up data is reassuring. Importantly, most people who have had myocarditis and/or pericarditis due to other causes recover completely and have no ongoing impairment of cardiac function.25 Early data suggest this is likely for cases associated with mRNA COVID-19 vaccination.
Patients with myocarditis and/or pericarditis after an mRNA COVID-19 vaccine whose symptoms resolve quickly, who do not have any arrhythmia associated with the acute myocarditis, and who have not had prolonged impairment of ventricular systolic function, should be followed up by a specialist for at least 12 months. There will usually be some restriction of exercise (particularly strenuous exercise or competitive sport) if they have confirmed myocarditis.
For any patient who is found to have a persisting abnormality, e.g. heart block or ventricular tachycardia, persisting ventricular dysfunction, or persisting abnormalities on a cardiac MRI (where applicable), follow-up should be extended in consultation with their treating specialist.
Reporting adverse events
Suspected cases of myocarditis or pericarditis following a COVID-19 vaccine should be reported to your jurisdiction vaccine safety service, with details available at the Therapeutic Goods Administration website.
More information
- CSANZ: csanz.edu.au/
- https://www.predict.org.au/chest-pain-guideline/
- Australian Product Information on Comirnaty and Spikevax COVID-19 vaccines, available on the TGA website.
- Department of Health: health.gov.au/covid19-vaccines
- Brighton Collaboration case definitions of myocarditis and pericarditis are available at https://brightoncollaboration.us/myocarditis-case-definition-update/.